Abstract
Approximately 65% of patients with DLBCL achieve a complete response (CR) with standard chemoimmunotherapy. Whilst the revised international prognostic index (R-IPI) has been shown to be predictive of progression-free survival (PFS), with 90% of those in the very good group (IPI 0) remaining progression-free compared to 50% in the poor risk group (IPI 3-5), the separation of the curves is most marked during the first year due to patients with refractory disease or early relapse accounting for the majority of the differences.
There are no internationally set criteria for routine follow-up for patients with DLBCL in remission, although numerous national bodies suggest follow-up for 5 years or longer with the focus primarily being on early detection of relapse. For example, NCCN recommends clinical review and bloods every 3-6 months for 5 years.
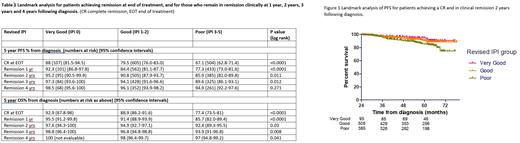
As part of a strategy to analyse the value of continued routine follow-up of all patients for 5 years, we performed landmark analysis of prospectively collected data from two large frontline clinical trials to see whether R-IPI remained predictive of relapse risk in patients who had achieved a CR on CT imaging by international standardised criteria and remained in clinical remission 1, 2, 3 and 4 years after diagnosis.
The R-CHOP14 versus 21 study demonstrated no improvement in outcome for patients with a more intensified treatment strategy. A total of 1080 patients were recruited of whom 607 out of 1002 assessable (61%) achieved a CR following completion of therapy. Routine scans were performed at 3 months following chemotherapy completion and at 12 months as part of the protocol, and then patients were routinely monitored clinically for relapse. REMoDL-B randomised 918 patients between R-CHOP versus R-CHOP plus bortezomib and again, no difference in outcome was seen with the addition of bortezomib. Across both arms, 61% achieved a CR. CT scans were performed routinely at end of treatment and at 1 year following completion of therapy.
Landmark analysis demonstrated a statistically significant difference in PFS based on R-IPI for patients still in clinical remission up to 3 years following diagnosis, however this difference was not apparent at 4 years (Table 1). Despite this statistical significance up to 3 years, the actual difference in PFS in earlier landmark analyses was small. For example, for patients still in remission clinically 2 years post diagnosis, the 5-year PFS was 85.9% (82.0-89.8), 90.8% (87.9-93.7) and 95.2% (90.5-99.9) for the poor, good and very good R-IPI groups respectively (Figure 1). 5-year overall survival (OS) was 92.4% (89.3-95.5), 94.9% (92.7-97.1) and 97.6% (94.3-100).
To assess the annual rate of relapse according to R-IPI group, patients who achieve a CR and were not censored for the following year were analysed to assess the rate of relapse per year. The rate of relapse in the first year was 4% (n=107) for those in the very good R-IPI group, 6% (n=595) in the good and 13% (n=496) in the poor risk group, however for the 3rd year the rates were 2% (n=86), 4% (n=455), and 4% (n=342) respectively and remained at this level for the 5th year. Thus despite a statistically significant landmark PFS difference by R-IPI group at 3 years remission, when assessing the relapse rate for the following year according to R-IPI, there was not a meaningful clinical difference.
The value of routine follow-up of patients in remission for relapse detection is in part related to the ability of the "tests” performed to detect relapse and also the rate of relapse. Our current tools for detecting relapse are either associated with low sensitivity or specificity or associated with risks such as excess radiation for repeated radiological examinations. Given the low incidence of relapse at any time post completion of therapy in the very good R-IPI group who achieve remission and in those in the other R-IPI groups following 2 years in remission, the positive predictive value of any test performed to detect relapse will be low and raises doubt as to whether it is cost effective to continue to follow patients up routinely. Instead this evidence supports consideration of a patient initiated follow-up strategy, where patients are educated to seek advice should they develop symptoms suggestive of relapse. Furthermore, this supports the consideration of focusing research for other more sensitive methods for relapse detection such as ctDNA on patients in the highest R-IPI group.
Disclosures
El-Sharkawi:Abbvie: Consultancy, Honoraria; Astex: Consultancy; AstraZeneca: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Gilead: Honoraria; Janssen: Consultancy, Honoraria; Kyowa Kiirin: Consultancy; Lilly: Consultancy; Novartis: Honoraria; Roche: Consultancy, Honoraria; Takeda: Honoraria. Thompson:Abbvie: Consultancy, Research Funding. Johnson:Epizyme: Consultancy, Research Funding; Janssen: Consultancy; Oncimmune: Consultancy; Boehringer Ingelheim: Consultancy; BMS: Honoraria; Celgene: Honoraria; Genmab: Honoraria; Incyte: Honoraria; Kite Pharma: Honoraria; Kymera: Honoraria; MorphoSys: Honoraria; Novartis: Honoraria; Takeda: Honoraria. Linch:Autolus: Consultancy. Iyengar:Lilly: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; AbbVie: Other: conference support; Kite Gilead: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: conference support, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees. Davies:Abbvie: Membership on an entity's Board of Directors or advisory committees; GSK: Other: Research support; Incyte: Membership on an entity's Board of Directors or advisory committees; Ascerta Pharma/Astra Zeneca: Honoraria, Other: Research support; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Kite, a Gilead company: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support. Travel to scientific conferences, Research Funding; Celegne (a Bristol Myers Squibb company): Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences, Research Funding. Cunningham:MedImmune: Research Funding; AstraZeneca: Research Funding; Clovis: Research Funding; Lilly: Research Funding; 4SC: Research Funding; Bayer: Research Funding; Celgene: Research Funding; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal